VRLA battery or valve-regulated lead acid is a type of lead acid battery that promotes minimum to no water addition maintenance, sealed in a structure with no acid leaks and no mists. It has a one-way valve to allow the escape of gas formed inside above a predetermined pressure value, preventing the VRLA battery from exploding. The valve does not allow atmospheric air to sip inside.
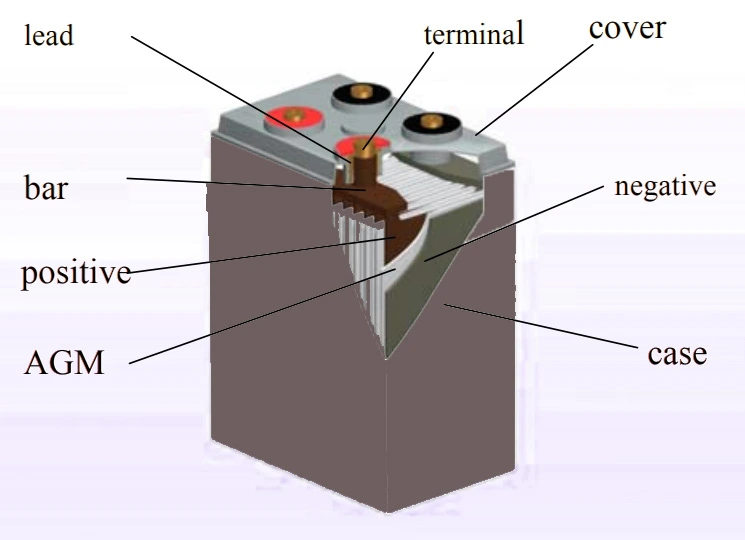
Table of Contents
Principle of operation of VRLA battery
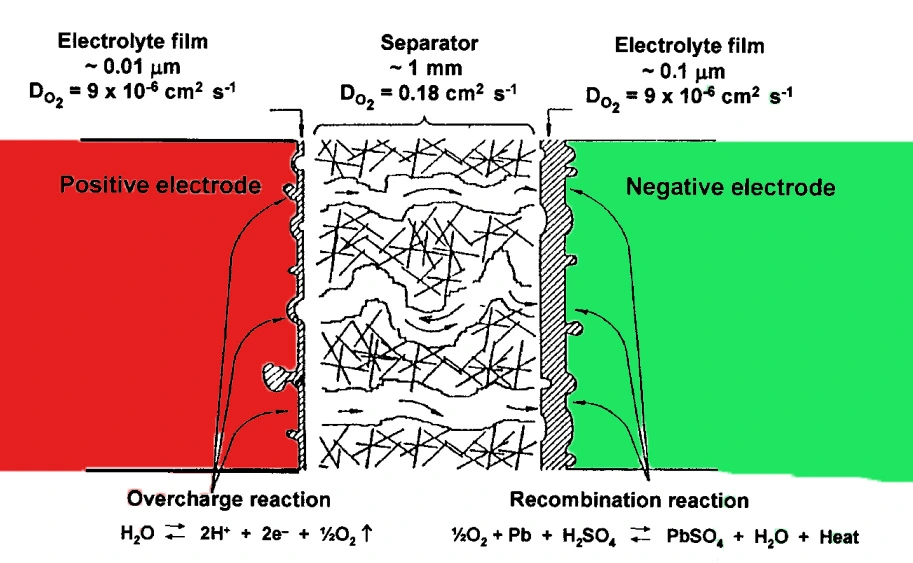
The VRLA battery is carefully designed so that it operates by means of an internal oxygen cycle also known as the oxygen-recombination cycle. Oxygen which is evolved in the latter stages of charging and overcharging, of the positive electrode, i.e.,
![]() (3)
(3)
transfers through a gaseous space to the negative electrode where it is reduced and recombined to form water:
![]() (4)
(4)
Two other reactions must be considered during the charging of the VRLA cell which are, the evolution of hydrogen at the negative plate of the cell and the corrosion of the positive grid:
![]() (5)
(5)
![]() (6)
(6)
Thus, the charging of a VRLA cell becomes more complex than the charging of the flooded counterpart cell. During the charging of a VRLA cell, thermodynamic/kinetic conditions allow the progress of six separate reactions at significant rates:
two charging reactions
![]() (1)
(1)
![]() (2)
(2)
and four secondary reactions 3 to 6
The oxygen cycle, which is defined by reactions 3 and 4, alters the potential of the negative electrode to a less negative value and thus decreases the rate of hydrogen evolution to a lower level which is much less than in the older, flooded design of the battery.
A one-way, pressure relief valve is fixed to the enclosure which ensures safe pressure within the VRLA battery even if the hydrogen is produced and hence is the term valve-regulated.
Since the plate is simultaneously on a charge, the lead sulfate produced is immediately reduced to lead via the reverse of reaction 2. This restores the chemical balance of the cell, i.e., in stoichiometric terms, the net sum of reaction 3,4 and the reverse of reaction 2 is zero.
Thus, part of the energy supplied to the cell is consumed by the oxygen-recombination cycle and is converted into heat rather than chemical energy. As long as the overcharging current remains moderate, the charge and recombination reactions can remain in equilibrium and little net gas will be generated.
For example, let us consider a flooded lead acid cell charged to a constant voltage of 2.45 V, the current at the positive electrode is consumed principally by the evolution of oxygen (IO2) and by grid corrosion (Ic), and is balanced by that consumed by evolution of hydrogen at the negative electrode (IH2).
With a valve-regulated cell, the oxygen reduction (IO2-red) shifts the potential of the negative electrode (delta-V) and the rate of hydrogen evolution (IH2) becomes much reduced, but is not eliminated completely, compared to the flooded system. The high recombination efficiency and no loss of oxygen from the cell, the rate of hydrogen evolution is therefore balanced by the rate of positive-grid corrosion. In general, the rate at which oxygen recombines at the negative plate is a complex function of the cell design, the operating conditions, and the overcharge function.
The separator, or more precisely the gas space employed to restrict the electrolyte, is the main component of the valve-regulated lead–acid VRLA cell as it gives the means for valve-regulated operation. The electrolyte must have ready access to the active material on the positive and negative plates, and a free passage of oxygen from the positive plate to the negative plate during charging. This requirement demands gas channels between both the plates of the cell because the coefficient of diffusion of oxygen through the aqueous electrolyte phase is some four orders smaller than that through the air.
Also, the separator system must offer all the properties necessary for the flooded version of the lead–acid cell, from which the VRLA design has been developed. These properties include:
- chemical stability in the acid environment,
- electronic insulation,
- sufficiently open porosity for allowing low ionic resistance through the electrolyte phase,
- good dimensional stability, and
- adequate strength for resisting puncture by lead ‘dendrites’.
There are basically two designs for providing the space to the gas in VRLA cells. One of the designs has the electrolyte immobilized as a gel, and the other has the electrolyte held in the AGM separator. The gas either passes through fissures present in the gel, or through AGM channels.
AGM Absorbent Glass Mat type VRLA battery
In the AGM process, a very fine glass wool separator is used, having a porosity of more than 93%, and absorbing sufficient electrolyte from the reaction. It makes sure that no flow of electrolytes occurs inside the VRLA battery. When the separator is absorbed fully with electrolyte, it still maintains 10% of the pores for an efficient combination of oxygen precipitating from the positive electrode and compound H2o from the negative electrode, which makes the cell attain the sealing effect.
Advantages of AGM
- The cell’s self-discharge rate is below 2% at 25 degrees Celsius
- These batteries have good charging efficiency
- The resistance is less at 0.2 to 0.9m Ω because of its tight assembly hence allowing for the high current discharge
- These batteries have a very good discharge performance at low temperatures.
Gel separator type VRLA battery
The principle of sealing in this type of VRLA battery is the same as AGM separator VRLA battery with respect to the oxygen cycle. But here the oxygen from the positive electrode is not transmitted through the pores to the negative electrode for recombination. The recombination is achieved through the cracks of the colloids in the gel. These cracks form over time as the gel shrinks making the oxygen recombination efficiency better which remains low in the initial stage.
Characteristics of gel separator
- Efficient liquid design promoting good characteristics of deep discharge recovery
- At higher temperatures, the performance is better than the AGM separator VRLA battery.
- These batteries are not suitable for high discharge rates at low temperatures.
- Not suitable for thin plate design
- More sensitive to overcharging.
- The gel may flow out if the VRLA battery is tilted.
Applications of VRLA battery
The VRLA battery finds its applications in the following
- Uninterruptible power supply for providing backup power to critical power requirements.
- Extensively used in the telecommunication sectors for powering the electronics and ensure continuous operation
- Used in power substations as an auxiliary source of power for control and relay circuits.
- Used in marine applications for powering navigation systems etc.
This article is a part of the Energy storage and reactive power compensation page, where other articles related to the topic are discussed in details.
